Ionic and Covalent Compounds Directed Reading Answer Key
In ordinary chemical reactions, the nucleus of each atom (and thus the identity of the element) remains unchanged. Electrons, nonetheless, can exist added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements.
The nature of the bonny forces that hold atoms or ions together within a compound is the basis for classifying chemical bonding. During the formation of some compounds, atoms proceeds or lose electrons, and grade electrically charged particles called ions (Effigy \(\PageIndex{1}\)). When this occurs, ionic bonds result. Ionic bonds are electrostatic forces of allure, that is, the bonny forces experienced betwixt objects of opposite electric accuse (in this case, cations and anions). When electrons are "shared" and molecules class, covalent bonds issue. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located betwixt the atoms. Compounds are classified as ionic or molecular (covalent) on the basis of the bonds present in them.
Figure \(\PageIndex{1}\): (a) A sodium atom (Na) has equal numbers of protons and electrons (11) and is uncharged. (b) A sodium cation (Na+) has lost an electron, so it has one more proton (xi) than electrons (10), giving it an overall positive charge, signified past a superscripted plus sign.
Ion Formation
Yous can use the periodic table to predict whether an cantlet will class an anion or a cation, and y'all can often predict the charge of the resulting ion. Atoms of many main-group metals (not the transition metals) lose enough electrons to exit them with the same number of electrons equally an atom of the preceding noble gas. To illustrate, an atom of an alkali metallic (group 1) loses i electron and forms a cation with a 1+ charge; an alkaline globe metallic (group 2) loses two electrons and forms a cation with a 2+ charge, and so on. For example, a neutral calcium cantlet, with twenty protons and twenty electrons, readily loses two electrons. This results in a cation with 20 protons, xviii electrons, and a 2+ accuse. It has the aforementioned number of electrons as atoms of the preceding element of group 0, argon, and is symbolized Ca2+. The proper noun of a metal ion is the same as the name of the metal atom from which it forms, so Ca2+ is called a calcium ion.
When atoms of nonmetal elements grade ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic tabular array. Atoms of grouping 17 gain i electron and form anions with a 1− charge; atoms of grouping 16 proceeds 2 electrons and form ions with a 2− accuse, and so on. For example, the neutral bromine cantlet, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. This results in an anion with 35 protons, 36 electrons, and a 1− charge. It has the aforementioned number of electrons equally atoms of the next noble gas, krypton, and is symbolized Br−. (A discussion of the theory supporting the favored condition of noble gas electron numbers reflected in these predictive rules for ion formation is provided in a later chapter of this text.)
Note the usefulness of the periodic tabular array in predicting likely ion formation and charge (Figure \(\PageIndex{2}\)). Moving from the far left to the right on the periodic tabular array, main-group elements tend to class cations with a accuse equal to the group number. That is, group one elements form ane+ ions; group 2 elements form 2+ ions, so on. Moving from the far right to the left on the periodic table, elements oftentimes form anions with a negative charge equal to the number of groups moved left from the noble gases. For example, grouping 17 elements (1 group left of the noble gases) course 1− ions; group xvi elements (ii groups left) form 2− ions, and so on. This trend tin be used every bit a guide in many cases, but its predictive value decreases when moving toward the center of the periodic table. In fact, transition metals and some other metals often exhibit variable charges that are not predictable by their location in the table. For example, copper can form ions with a 1+ or 2+ charge, and fe can course ions with a 2+ or three+ charge.
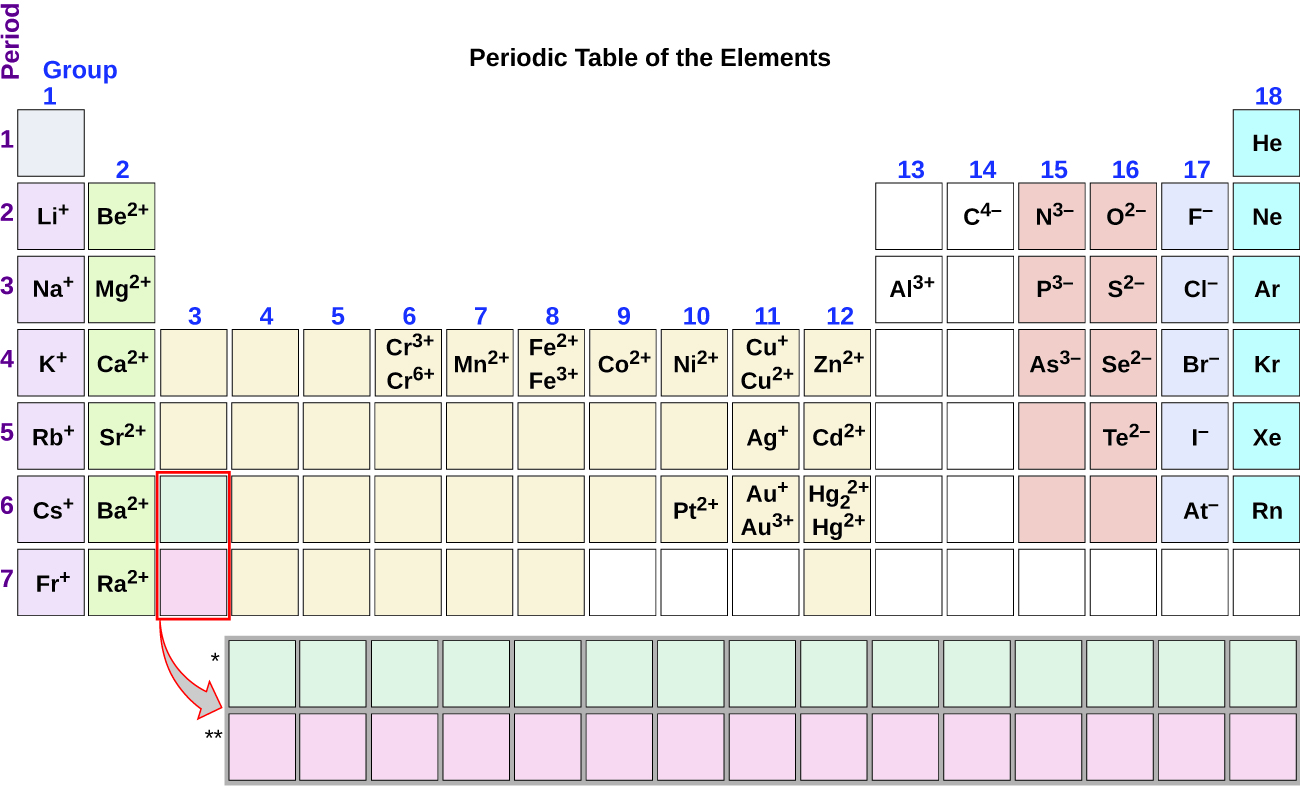
Effigy \(\PageIndex{ii}\): Some elements showroom a regular pattern of ionic accuse when they form ions.
Example \(\PageIndex{i}\): Composition of Ions
An ion found in some compounds used as antiperspirants contains thirteen protons and 10 electrons. What is its symbol?
Solution
Considering the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Knowing this lets us utilize the periodic table to place the element equally Al (aluminum). The Al cantlet has lost 3 electrons and thus has three more positive charges (13) than it has electrons (x). This is the aluminum cation, Aliii+.
Exercise \(\PageIndex{one}\)
Give the symbol and name for the ion with 34 protons and 36 electrons.
- Answer
-
Se2−, the selenide ion
Example \(\PageIndex{2}\): Germination of Ions
Magnesium and nitrogen react to form an ionic chemical compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them.
Solution
Magnesium's position in the periodic table (grouping 2) tells us that it is a metal. Metals form positive ions (cations). A magnesium atom must lose two electrons to accept the same number electrons every bit an atom of the previous noble gas, neon. Thus, a magnesium atom will class a cation with 2 fewer electrons than protons and a charge of two+. The symbol for the ion is Mg2+, and information technology is called a magnesium ion.
Nitrogen's position in the periodic table (group 15) reveals that it is a nonmetal. Nonmetals grade negative ions (anions). A nitrogen cantlet must gain three electrons to have the same number of electrons as an atom of the post-obit noble gas, neon. Thus, a nitrogen cantlet will class an anion with three more electrons than protons and a charge of three−. The symbol for the ion is North3−, and it is called a nitride ion.
Exercise \(\PageIndex{2}\)
Aluminum and carbon react to form an ionic chemical compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them.
- Answer
-
Al volition form a cation with a charge of 3+: Al3+, an aluminum ion. Carbon will form an anion with a charge of 4−: Civ−, a carbide ion.
The ions that we have discussed so far are called monatomic ions , that is, they are ions formed from only i cantlet. We besides find many polyatomic ions . These ions, which act every bit discrete units, are electrically charged molecules (a group of bonded atoms with an overall charge). Some of the more of import polyatomic ions are listed in Table \(\PageIndex{1}\). Oxyanions are polyatomic ions that comprise one or more oxygen atoms. At this point in your study of chemistry, y'all should memorize the names, formulas, and charges of the almost common polyatomic ions. Because you will apply them repeatedly, they will before long become familiar.
| Proper noun | Formula | Related Acid | Formula |
|---|---|---|---|
| ammonium | \(\ce{NH4+}\) | ||
| hydronium | \(\ce{H_3O^+}\) | ||
| oxide | \(\ce{O^{ii-}}\) | ||
| peroxide | \(\ce{O_2^{2-}}\) | ||
| hydroxide | \(\ce{OH^-}\) | ||
| acetate | \(\ce{CH_3COO^-}\) | acetic acid | \(\ce{CH_3COOH}\) |
| cyanide | \(\ce{CN^-}\) | hydrocyanic acid | \(\ce{HCN}\) |
| azide | \(\ce{N_3^-}\) | hydrazoic acid | \(\ce{HN_3}\) |
| carbonate | \(\ce{CO_3^{two-}}\) | carbonic acid | \(\ce{H_2CO_3}\) |
| bicarbonate | \(\ce{HCO_3^-}\) | ||
| nitrate | \(\ce{NO_3^-}\) | nitric acid | \(\ce{HNO_3}\) |
| nitrite | \(\ce{NO_2^-}\) | nitrous acid | \(\ce{HNO_2}\) |
| sulfate | \(\ce{SO_4^{2-}}\) | sulfuric acid | \(\ce{H_2SO_4}\) |
| hydrogen sulfate | \(\ce{HSO_4^-}\) | ||
| sulfite | \(\ce{SO_3^{2-}}\) | sulfurous acid | \(\ce{H_2SO_3}\) |
| hydrogen sulfite | \(\ce{HSO_3^-}\) | ||
| phosphate | \(\ce{PO_4^{3-}}\) | phosphoric acrid | \(\ce{H_3PO_4}\) |
| hydrogen phosphate | \(\ce{HPO_4^{2-}}\) | ||
| dihydrogen phosphate | \(\ce{H_2PO_4^-}\) | ||
| perchlorate | \(\ce{ClO_4^-}\) | perchloric acid | \(\ce{HClO_4}\) |
| chlorate | \(\ce{ClO_3^-}\) | chloric acid | \(\ce{HClO_3}\) |
| chlorite | \(\ce{ClO_2^-}\) | chlorous acid | \(\ce{HClO_2}\) |
| hypochlorite | \(\ce{ClO^-}\) | hypochlorous acid | \(\ce{HClO}\) |
| chromate | \(\ce{CrO_4^{2-}}\) | chromic acrid | \(\ce{H_2CrO_4}\) |
| dichromate | \(\ce{Cr_2O_7^{ii-}}\) | dichromic acid | \(\ce{H_2Cr_2O7}\) |
| permanganate | \(\ce{MnO_4^-}\) | permanganic acrid | \(\ce{HMnO_4}\) |
Note that in that location is a system for naming some polyatomic ions; -ate and -ite are suffixes designating polyatomic ions containing more or fewer oxygen atoms. Per- (brusque for "hyper") and hypo- (meaning "under") are prefixes meaning more oxygen atoms than -ate and fewer oxygen atoms than -ite, respectively. For example, perchlorate is \(\ce{ClO4-}\), chlorate is \(\ce{ClO3-}\), chlorite is \(\ce{ClO2-}\) and hypochlorite is ClO−. Unfortunately, the number of oxygen atoms corresponding to a given suffix or prefix is not consequent; for example, nitrate is \(\ce{NO3-}\) while sulfate is \(\ce{SO4^{two-}}\). This will be covered in more detail in the next module on nomenclature.
Ionic Compounds
When an element composed of atoms that readily lose electrons (a metal) reacts with an element composed of atoms that readily proceeds electrons (a nonmetal), a transfer of electrons usually occurs, producing ions. The compound formed past this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite accuse present in the chemical compound. For example, when each sodium atom in a sample of sodium metal (group 1) gives upwardly one electron to grade a sodium cation, Na+, and each chlorine atom in a sample of chlorine gas (group 17) accepts one electron to course a chloride anion, Cl−, the resulting compound, NaCl, is composed of sodium ions and chloride ions in the ratio of one Na+ ion for each Cl− ion. Similarly, each calcium atom (group two) tin can give up two electrons and transfer one to each of ii chlorine atoms to class CaCl2, which is equanimous of Ca2+ and Cl− ions in the ratio of i Ca2+ ion to two Cl− ions.
A compound that contains ions and is held together by ionic bonds is chosen an ionic chemical compound. The periodic table tin can help u.s. recognize many of the compounds that are ionic: When a metal is combined with one or more nonmetals, the compound is usually ionic. This guideline works well for predicting ionic compound formation for most of the compounds typically encountered in an introductory chemistry course.
You can often recognize ionic compounds considering of their properties. Ionic compounds are solids that typically melt at high temperatures and eddy at even higher temperatures. For instance, sodium chloride melts at 801 °C and boils at 1413 °C. (As a comparison, the molecular chemical compound water melts at 0 °C and boils at 100 °C.) In solid form, an ionic compound is not electrically conductive because its ions are unable to flow ("electricity" is the period of charged particles). When molten, nonetheless, it can carry electricity because its ions are able to motility freely through the liquid (Figure \(\PageIndex{3}\)).
Effigy \(\PageIndex{three}\): Sodium chloride melts at 801 °C and conducts electricity when molten. (credit: modification of work past Mark Blaser and Matt Evans)
In every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. Thus, ionic compounds are electrically neutral overall, even though they contain positive and negative ions. We can apply this observation to aid us write the formula of an ionic compound. The formula of an ionic chemical compound must have a ratio of ions such that the numbers of positive and negative charges are equal.
Example \(\PageIndex{3}\): Predicting the Formula of an Ionic Compound
The gemstone sapphire (Effigy \(\PageIndex{iv}\)) is more often than not a compound of aluminum and oxygen that contains aluminum cations, Al3+, and oxygen anions, O2−. What is the formula of this chemical compound?

Figure \(\PageIndex{4}\): Although pure aluminum oxide is colorless, trace amounts of iron and titanium give blue sapphire its feature color. (credit: modification of piece of work by Stanislav Doronenko)
Solution Because the ionic compound must be electrically neutral, it must accept the aforementioned number of positive and negative charges. Two aluminum ions, each with a accuse of iii+, would give us half-dozen positive charges, and three oxide ions, each with a charge of ii−, would give us six negative charges. The formula would be Al2O3.
Practise \(\PageIndex{3}\)
Predict the formula of the ionic compound formed between the sodium cation, Na+, and the sulfide anion, S2−.
- Answer
-
Na2S
Many ionic compounds incorporate polyatomic ions (Tabular array \(\PageIndex{1}\)) as the cation, the anion, or both. As with simple ionic compounds, these compounds must too exist electrically neutral, then their formulas tin can exist predicted by treating the polyatomic ions as discrete units. We employ parentheses in a formula to indicate a group of atoms that behave equally a unit. For example, the formula for calcium phosphate, i of the minerals in our bones, is Ca3(PO4)2. This formula indicates that at that place are 3 calcium ions (Caii+) for every two phosphate \(\left(\ce{PO4^{3-}}\right)\) groups. The \(\ce{PO4^{3-}}\) groups are discrete units, each consisting of ane phosphorus atom and 4 oxygen atoms, and having an overall accuse of 3−. The compound is electrically neutral, and its formula shows a total count of three Ca, two P, and eight O atoms.
Example \(\PageIndex{4}\): Predicting the Formula of a Chemical compound with a Polyatomic Anion
Baking pulverization contains calcium dihydrogen phosphate, an ionic chemical compound composed of the ions Ca2+ and \(\ce{H2PO4-}\). What is the formula of this compound?
Solution
The positive and negative charges must balance, and this ionic compound must be electrically neutral. Thus, we must have two negative charges to residuum the 2+ charge of the calcium ion. This requires a ratio of one Ca2+ ion to two \(\ce{H2PO4-}\) ions. Nosotros designate this past enclosing the formula for the dihydrogen phosphate ion in parentheses and adding a subscript two. The formula is Ca(H2PO4)ii.
Do \(\PageIndex{4}\)
Predict the formula of the ionic compound formed betwixt the lithium ion and the peroxide ion, \(\ce{O2^2-}\) (Hint: Utilise the periodic tabular array to predict the sign and the charge on the lithium ion.)
- Answer
-
Li2O2
Because an ionic compound is not fabricated up of single, discrete molecules, it may not be properly symbolized using a molecular formula. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its elective ions. For compounds containing only monatomic ions (such equally NaCl) and for many compounds containing polyatomic ions (such as CaSOiv), these formulas are just the empirical formulas introduced earlier in this chapter. However, the formulas for some ionic compounds containing polyatomic ions are not empirical formulas. For instance, the ionic compound sodium oxalate is comprised of Na+ and \(\ce{C2O4^2-}\) ions combined in a two:1 ratio, and its formula is written as NatwoC2O4. The subscripts in this formula are not the smallest-possible whole numbers, as each tin can be divided by 2 to yield the empirical formula, NaCOii. This is not the accustomed formula for sodium oxalate, still, every bit information technology does not accurately represent the compound'southward polyatomic anion, \(\ce{C2O4^ii-}\).
Molecular Compounds
Many compounds do not contain ions merely instead consist solely of discrete, neutral molecules. These covalent compounds (molecular compounds) outcome when atoms share, rather than transfer (proceeds or lose), electrons. Covalent bonding is an important and all-encompassing concept in chemical science, and information technology will be treated in considerable detail in a later chapter of this text. We can oft identify molecular compounds on the basis of their concrete properties. Under normal conditions, molecular compounds frequently be as gases, low-boiling liquids, and low-melting solids, although many of import exceptions exist.
Whereas ionic compounds are unremarkably formed when a metallic and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals. Thus, the periodic table can help united states of america recognize many of the compounds that are covalent. While we can use the positions of a compound's elements in the periodic table to predict whether information technology is ionic or covalent at this point in our study of chemical science, you lot should be aware that this is a very simplistic approach that does not business relationship for a number of interesting exceptions. Shades of gray be between ionic and molecular compounds, and you'll acquire more about those later.
Case \(\PageIndex{five}\): Predicting the Blazon of Bonding in Compounds
Predict whether the following compounds are ionic or molecular:
- KI, the chemical compound used as a source of iodine in table common salt
- H2O2, the bleach and disinfectant hydrogen peroxide
- CHCl3, the anesthetic chloroform
- Li2CO3, a source of lithium in antidepressants
Solution
- Potassium (group 1) is a metal, and iodine (grouping 17) is a nonmetal; KI is predicted to be ionic.
- Hydrogen (group i) is a nonmetal, and oxygen (group xvi) is a nonmetal; H2O2 is predicted to be molecular.
- Carbon (group xiv) is a nonmetal, hydrogen (group i) is a nonmetal, and chlorine (group 17) is a nonmetal; CHCliii is predicted to be molecular.
- Lithium (group 1) is a metallic, and carbonate is a polyatomic ion; Li2COthree is predicted to be ionic.
Practise \(\PageIndex{5}\)
Using the periodic table, predict whether the following compounds are ionic or covalent:
- And then2
- CaF2
- N2Hiv
- Alii(And then4)3
- Reply a
-
molecular
- Respond b
-
ionic
- Answer c
-
molecular
- Respond d
-
ionic
Summary
Metals (particularly those in groups ane and two) tend to lose the number of electrons that would leave them with the same number of electrons as in the preceding noble gas in the periodic table. By this ways, a positively charged ion is formed. Similarly, nonmetals (particularly those in groups 16 and 17, and, to a lesser extent, those in Grouping 15) tin can gain the number of electrons needed to provide atoms with the same number of electrons equally in the next noble gas in the periodic tabular array. Thus, nonmetals tend to form negative ions. Positively charged ions are called cations, and negatively charged ions are called anions. Ions tin be either monatomic (containing only one cantlet) or polyatomic (containing more than one atom).
Compounds that incorporate ions are called ionic compounds. Ionic compounds generally grade from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules (uncharged groups of atoms that behave every bit a unmarried unit), are called covalent compounds. Covalent compounds commonly form from two or more nonmetals.
Source: https://chem.libretexts.org/Courses/Bellarmine_University/BU%3A_Chem_103_(Christianson)/Phase_1%3A_Chemistry_Essentials/3%3A_Chemical_Compounds/3.5%3A_Ionic_and_Covalent_Compounds
0 Response to "Ionic and Covalent Compounds Directed Reading Answer Key"
Post a Comment